Ask an Expert
Back to Categories
What are the guidelines defining objective criteria for acceptable performance of the internal controls required to satisfy CAP checklist item FL.23737?
CAP checklist item FL.23737 requires that "The performance of reagents and staining procedures are verified by the use of positive controls." There are two types of controls:
1. External controls are an integral part of every laboratory assay as they "control" the overall assay performance including instrument settings, antibody performance, and specimen preparation, etc. External controls can be QC'd through certain cellular subset populations of patient specimens (bone marrow, peripheral blood, tissue) at least monthly. An approximate range of acceptable percentages and mean fluorescence intensity can be used to determine if the performance of the antibody meets the acceptability criteria as determined by the laboratory. Commercially available fixed control material can also be used, especially for antigens less frequently found on patient samples (e.g., CD103, CD25, etc).
2. Internal controls serve a different role, as they verify the assay performance in the patient sample. An example would be that the CD10 performance needs to be verified using external controls (e.g., CD10 staining on mature granulocytes, B lymphoblasts, or other QC material) prior to using this antibody for patient testing. Internal control cells (e.g., mature granulocytes in the patient sample) verify that the CD10 was actually added to the patient sample and thus verify the performance within the patient sample. Internal controls are represented by the variable number of residual normal cells in the patient's sample and it should be a part of the interpretation of the patient's dotplots. The flow cytometrist will evaluate the "diagnostic dotplots" to identify possible abnormalities within the patient's sample. A targeted design of "control dotplots" for each panel tube will ensure that the correct antibodies or cocktails were added and verify the performance of each antibody. The expected antibody performance on internal controls can then be documented for each patient. Examples of "control dotplots" with expected results are given below.
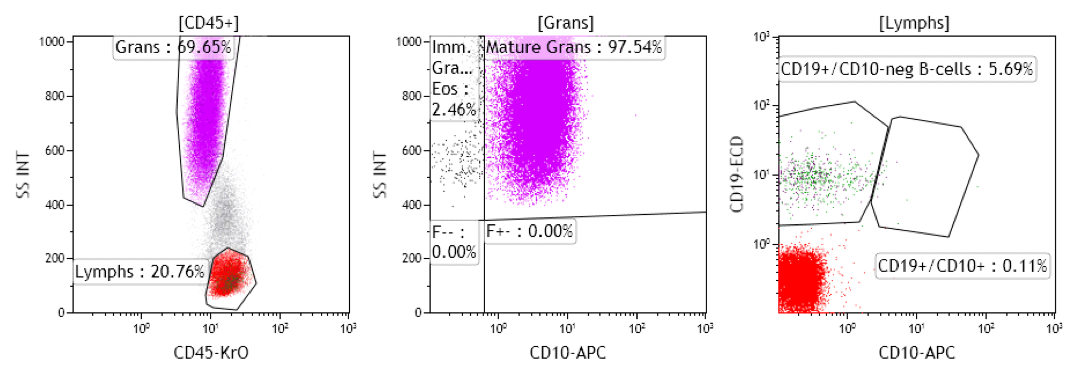
Figure 1: Peripheral blood should show CD10 expression on mature neutrophils, no expression on eosinophils or immature granulocytes, and no expression on mature B-cells.

Figure 2: Presentation of all PMTs vs SS allows for easy assessment of all WBC subsets as internal positive or negative controls.

Figure 3: Combination of gating (A, B, and D), diagnostic (C for PNH clone in granulocytes and E for PNH clone in monocytes), and control dotplots (F) in normal peripheral blood submitted for PNH testing. Lymphocytes are not target cells for PNH clone determination but serve as excellent internal control cells: B-cells are CD24+/FLAER+, T-cells are CD24-neg and FLAER+, NK cells are CD24-neg and FLAER dim+.
SUMMARY
It is recommended that each laboratory design a quality control plan for their antibodies using external controls and develops standards of acceptability. Some assays may require daily controls (CD4/CD8, CD34, etc). Others may include suggested ranges for percent positives, ranges for expected MFI as well as a visual inspection of dotplots (e.g., leukemia/lymphoma evaluation). This assessment should be performed at least monthly for most antibodies and at least semi-annually for rare antigens. Internal controls can be used for all panel tubes and targeted design of certain subsets may be used to verify positive or negative antibody staining. Cocktails should be used whenever possible as its use increases the confidence that all antibodies were added prior to patient testing. However, internal controls should be used to verify that the right cocktail was added.
Author: Andrea Illingworth
